
- Show results for
- Share
How To Use Colorimeter
Resource Description

Many biochemical experiments involve the measurements of compounds or groups of compounds presented in a complex mixture.
The most widely used method for determining the concentration of biochemical compounds is colorimetry.

Colorimetry is the measurement of the concentration of a particular compound (solute) in a colored solution (solvent). It is used extensively for identification and determination of concentrations of substances that absorb light.
During the experiment, scientists often need to measure quantities of a particular compound in a mixture or the concentration of a solution. Our eyes are not good enough to determine the concentration in colored solutions, that’s why the colorimetry is a quality and important part of the experiment.
For such investigations we need a certain device that is called a colorimeter.
What is colorimeter and its construction
Colorimeter is a device that is used to measure light absorbance (how much light is absorbed) and transmittance of light (how much light passes through) in a liquid by analyzing color intensity.
Popular Products
The earliest colorimeters relied on the human eye to match the color of a solution with that one of a series of colored discs. The results were too subjective and not particularly accurate.

A colorimeter is generally any tool that characterizes color samples to provide an objective measure of color characteristics.
In chemistry, the colorimeter is a device that determines the absorbance of a solution at a particular frequency (color) of visual light. Colorimeters make it possible to determine the concentration of a known solute since it has been proportional to the absorbance.
Colorimeters are color comparison tools that are often mistaken with spectrophotometers. To understand how colorimeter works, you should know its construction:
- Light source, which is usually an ordinary light bulb, that passes the light through the object.
- Lens with an aperture that can be adjusted;
- Filters that isolate specific wavelength to be applied to the sample;
- Cuvette is a bulb that holds the liquid;
- Photocell, which measures the light that has passed through the solution;
- The Galvanometer displays the output from the detector.
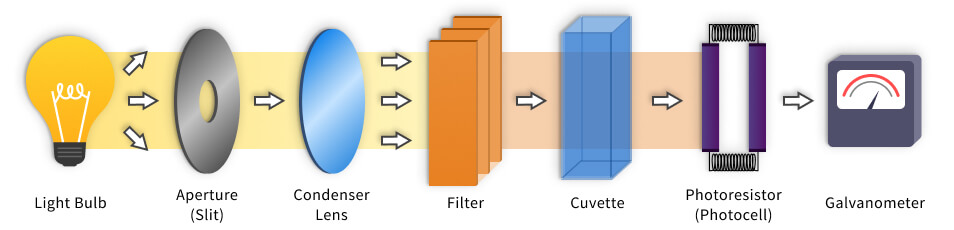
How does a colorimeter work?
Colorimeters rely on the Beer-Lambert's Law concept, which postulates that the absorbance of a substance is proportional to its concentration. For example, the more concentrated solution gives the higher absorbance reading. Many experiments in chemistry and biology are based on this concept. To obtain a Beer’s law curve, several standards (solutions of known concentration) are prepared, and their absorbance values are determined by using a Colorimeter.
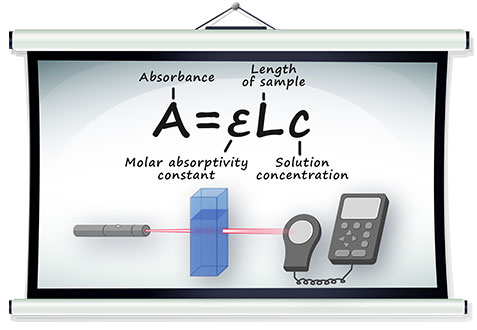
Beer-Lambert’s Law is written as:
A = absorbance;
c = concentration (mol/litre);
l = length of light path through the cell;
ε = molar absorption;
A = εlc;
To analyze color against an existing standard, the colorimeter sends light through the cuvette with liquid. The colorimeter’s lens and filter turn the beam of light into an isolated wavelength. The photocell calculates how much of the wavelength was absorbed, and the device returns the results on its digital display.
Difference between colorimeter and spectrophotometer
A spectrophotometer is a device that measures the intensity of light (the amount of photons) absorbed after it passes through solution.

Colorimeters and spectrophotometers are two of the most advanced color measurement devices. Although they are closely common, they each have unique pros and cons that make them best suited to different types of measurements. However, the biggest difference between the two devices is in facility and usage.
Spectrophotometer is more powerful and can make different types of measurements such as spectral data. That is why it is used for experiments in laboratories. Colorimeters are simpler and used in manufacturing for quality control. Spectrophotometers are more expensive than colorimeters because of their deeper function. Colorimeters aren’t as accurate as spectrophotometers.
Tips for getting accurate results
- Sample cuvette should be made of glass or plastic for visible range of light.
- The wavelength at which maximum absorption occurs, for the solution in question, should be selected for the analysis. It has been shown experimentally that the absorbance at this wavelength is more stable than at other wavelengths.
- The solutions should be properly diluted to avoid high absorbance value which could lead to the wrong calculation of the concentration.
- Soft light, either emerging from within the colorimeter due to an issue with the optics or from outside due to inadequate sealing would influence the reading in the meters.







